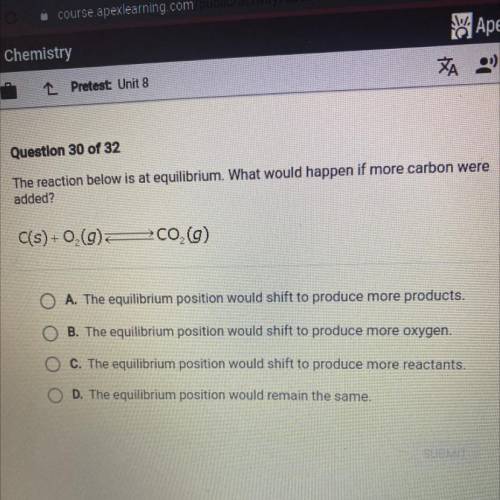
The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,(9...

The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,(9)
700 (9)
A. The equilibrium position would shift to produce more products.
B. The equilibrium position would shift to produce more oxygen.
C. The equilibrium position would shift to produce more reactants.
D. The equilibrium position would remain the same.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Questions

Mathematics, 23.10.2020 01:01

Computers and Technology, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01



Mathematics, 23.10.2020 01:01


Chemistry, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01



Biology, 23.10.2020 01:01


History, 23.10.2020 01:01



