A. Keg


Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1
You know the right answer?
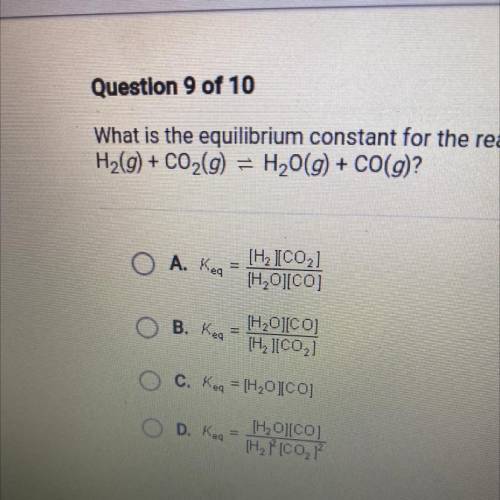
What is the equilibrium constant for the reaction
H2(g) + CO2(g) = H2O(g) + CO(g)?
A. Keg
A. Keg
Questions







Mathematics, 08.10.2021 21:30

Mathematics, 08.10.2021 21:30


History, 08.10.2021 21:30

History, 08.10.2021 21:30

Mathematics, 08.10.2021 21:30


History, 08.10.2021 21:30

Biology, 08.10.2021 21:30





Mathematics, 08.10.2021 21:30