Gas Laws Review and Intro to thermochemistry
4 of 30
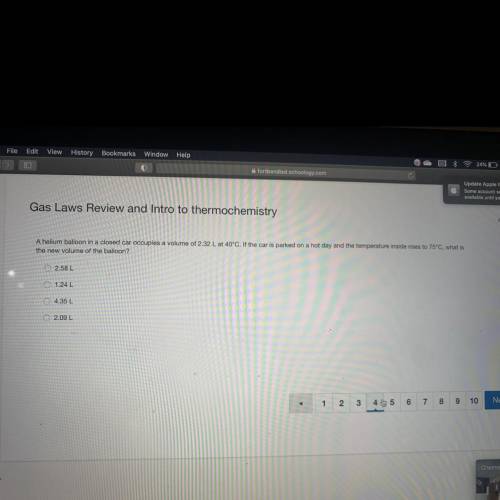
A helium balloon in a closed car occupie...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2
You know the right answer?
Questions

History, 02.04.2020 06:25



Mathematics, 02.04.2020 06:25

Mathematics, 02.04.2020 06:25


Mathematics, 02.04.2020 06:25



Mathematics, 02.04.2020 06:25

Mathematics, 02.04.2020 06:25

Mathematics, 02.04.2020 06:25


Social Studies, 02.04.2020 06:25



Chemistry, 02.04.2020 06:25


Mathematics, 02.04.2020 06:25