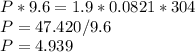Chemistry, 17.05.2021 17:40 loveyeti106838
At 304 K, a 9.6 L tank contains 1.9 moles of H2 gas under an unknown pressure in atm. What is the pressure of the gas in the tank? (R = 0.0821 L*atm/mol*K)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
At 304 K, a 9.6 L tank contains 1.9 moles of H2 gas under an unknown pressure in atm. What is the pr...
Questions


Mathematics, 11.02.2020 08:09



English, 11.02.2020 08:11


Biology, 11.02.2020 08:11


Mathematics, 11.02.2020 08:12

Health, 11.02.2020 08:21

Biology, 11.02.2020 08:21

Mathematics, 11.02.2020 08:21


Mathematics, 11.02.2020 08:21

Mathematics, 11.02.2020 08:22



History, 11.02.2020 08:22

History, 11.02.2020 08:22

Mathematics, 11.02.2020 08:22