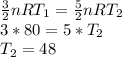Heat is added to two identical samples of a monatomic ideal gas. In the first sample, the heat is added while the volume of the gas is kept constant, and the heat causes the temperature to rise by 80 K. In the second sample, an identical amount of heat is added while the pressure (but not the volume) of the gas is kept constant. By how much does the temperature of this sample increase

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
Heat is added to two identical samples of a monatomic ideal gas. In the first sample, the heat is ad...
Questions


Chemistry, 20.05.2020 09:58

Biology, 20.05.2020 09:58

Health, 20.05.2020 09:58

History, 20.05.2020 09:58

Biology, 20.05.2020 09:58

Mathematics, 20.05.2020 09:58


Mathematics, 20.05.2020 09:58

Mathematics, 20.05.2020 09:58



History, 20.05.2020 09:58





World Languages, 20.05.2020 09:58


History, 20.05.2020 09:58