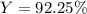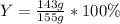Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
For the reaction 2Na + Cl2 2NaCl, calculate the percent yield if
155.0 g of chlorine (Cl2) should b...
Questions




History, 11.09.2019 00:30



Mathematics, 11.09.2019 00:30


Mathematics, 11.09.2019 00:30





Mathematics, 11.09.2019 00:30



Mathematics, 11.09.2019 00:30

History, 11.09.2019 00:30


English, 11.09.2019 00:30