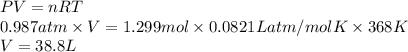Chemistry, 15.05.2021 02:00 leomessifanboy678
Hydrogen sulfide gas can be produced from the reaction of aluminum sulfide and water according to the following equation: Al2S3(s) 6H2O(l) > 3 H2S(g) 2Al(OH)3(aq) What volume of hydrogen sulfide gas is produced if 65.0 g Al2S3 reacts at 90.0 oC and 0.987 atm,

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Hydrogen sulfide gas can be produced from the reaction of aluminum sulfide and water according to th...
Questions






Computers and Technology, 22.06.2019 00:50









History, 22.06.2019 00:50

Mathematics, 22.06.2019 00:50




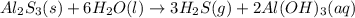
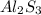 is given as 65.0 g. Hence, moles of
is given as 65.0 g. Hence, moles of 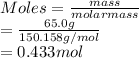
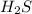 . Hence, moles of
. Hence, moles of 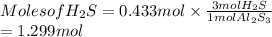
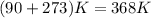 . Therefore, volume of hydrogen sulfide is calculated as follows.
. Therefore, volume of hydrogen sulfide is calculated as follows.