
Chemistry, 14.05.2021 23:20 saltyimps3
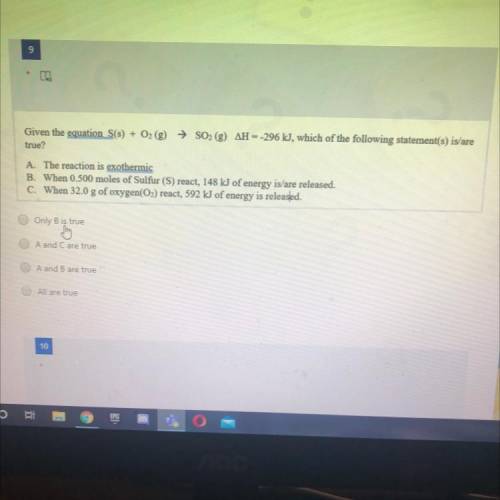
Given the equation S(s) + O2 (g) → SO2 (g) AH --296 kJ, which of the following statement(s) is/are
true?
A. The reaction is exothermic
B. When 0.500 moles of Sulfur (S) react, 148 kJ of energy is/are released.
C. When 32.0 g of oxygen(O2) react, 592 kJ of energy is released.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 21.06.2019 16:40
Who is better, messi or cristiano, i need this for a chemistry class. asap
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2
You know the right answer?
Given the equation S(s) + O2 (g) → SO2 (g) AH --296 kJ, which of the following statement(s) is/are...
Questions

Mathematics, 12.12.2020 15:50

Computers and Technology, 12.12.2020 15:50




Mathematics, 12.12.2020 15:50







English, 12.12.2020 15:50

History, 12.12.2020 15:50


Mathematics, 12.12.2020 15:50




English, 12.12.2020 15:50



