

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
A 2.5 M solution of a weak acid is prepared. Using a pH meter, the pH is measured to be 5.1. Calcula...
Questions




Spanish, 20.07.2019 06:30



Biology, 20.07.2019 06:30

Mathematics, 20.07.2019 06:30



Mathematics, 20.07.2019 06:30




Mathematics, 20.07.2019 06:30





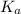 , of this weak acid.
Show your work
, of this weak acid.
Show your work

