
Chemistry, 14.05.2021 20:10 jazzycintron14
Geologists can estimate the age of rocks by their uranium-238 content. The uranium is incorporated in the rock as it hardens and then decays with first-order kinetics and a half-life of 4.5 billion years. A rock is found to contain 83.9% of the amount of uranium-238 that it contained when it was formed. (The amount that the rock contained when it was formed can be deduced from the presence of the decay products of U-238.) How old is the Rock?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

You know the right answer?
Geologists can estimate the age of rocks by their uranium-238 content. The uranium is incorporated i...
Questions


Mathematics, 12.02.2020 04:45



Mathematics, 12.02.2020 04:45







Mathematics, 12.02.2020 04:45

History, 12.02.2020 04:45




History, 12.02.2020 04:45



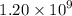 years
years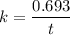
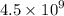 years
years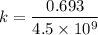
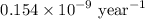
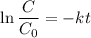
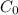 = initial amount
= initial amount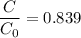
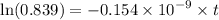
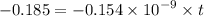
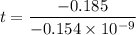
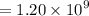 years
years


