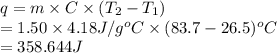Chemistry, 14.05.2021 18:40 shanicar33500
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required to heat 1.50 g of water from 26.5oC to 83.7oC. (Ignore significant figures for this problem.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required...
Questions


English, 18.08.2019 18:30

Mathematics, 18.08.2019 18:30


Mathematics, 18.08.2019 18:30

Geography, 18.08.2019 18:30

Mathematics, 18.08.2019 18:30

Biology, 18.08.2019 18:30

History, 18.08.2019 18:30

Mathematics, 18.08.2019 18:30


History, 18.08.2019 18:30

Physics, 18.08.2019 18:30

Mathematics, 18.08.2019 18:30

Mathematics, 18.08.2019 18:30


Physics, 18.08.2019 18:30

History, 18.08.2019 18:30


Social Studies, 18.08.2019 18:30

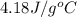
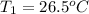
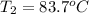
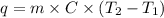
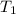 = initial temperature
= initial temperature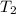 = final temperature
= final temperature