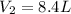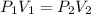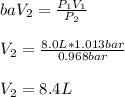Chemistry, 14.05.2021 15:30 joshawajackson
A balloon is inflated to a volume of 8.0 L on a day when the atmospheric pressure is 1.013 bar . The next day, a storm front arrives, and the atmospheric pressure drops to 0.968 bar . Assuming the temperature remains constant, what is the new volume of the balloon, in liters

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
A balloon is inflated to a volume of 8.0 L on a day when the atmospheric pressure is 1.013 bar . The...
Questions


Biology, 12.01.2021 19:50

Mathematics, 12.01.2021 19:50

Arts, 12.01.2021 19:50

Mathematics, 12.01.2021 19:50


Mathematics, 12.01.2021 19:50



Mathematics, 12.01.2021 19:50


Social Studies, 12.01.2021 19:50


Geography, 12.01.2021 19:50


English, 12.01.2021 19:50


English, 12.01.2021 19:50


Social Studies, 12.01.2021 20:00