
Chemistry, 14.05.2021 14:00 rubiim9610
What is the density of a gas at STP that has a molar mass of 50.0 g/mol?
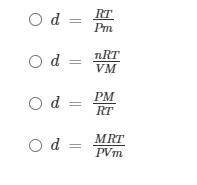
Student A wrote some preliminary notes: PV = nRT; n = m/M; and d = m/V
known: unknown
T = 273 K d = g/cm3
P = 1 atm
R = 0.08125 atm
M = 50.0 g/mol
If PV = nRT and n = m/M, what is the density of the ideal gas?
Help Student A find the equation to use to solve this problem:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2


Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
What is the density of a gas at STP that has a molar mass of 50.0 g/mol?
Student A wrote some preli...
Questions


English, 28.05.2021 01:00


Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00


English, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00






Biology, 28.05.2021 01:00



Mathematics, 28.05.2021 01:00

Business, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00



