
Chemistry, 14.05.2021 06:20 butterflyrhodes01
Considere la combustión del monóxido de carbono (CO) en oxígeno gaseoso: 2CO(g) + O2(g) → 2CO2(g) Si la reacción se inicia con 3.60 moles de CO, calcule el número de moles de CO2 que se producen si hay suficiente oxígeno para reaccionar con todo el CO.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Considere la combustión del monóxido de carbono (CO) en oxígeno gaseoso: 2CO(g) + O2(g) → 2CO2(g) S...
Questions

Geography, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01




English, 20.09.2020 14:01

Business, 20.09.2020 14:01










Biology, 20.09.2020 14:01

Spanish, 20.09.2020 14:01

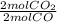 = 3.60 moles CO₂
= 3.60 moles CO₂

