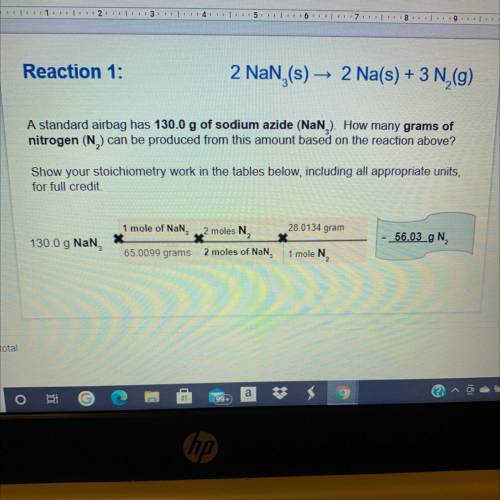
Reaction 2:
10 Na, + 2 KNO
3 (s)
KO + 5 Nao +
2 (9)
Based on the mass of so...

Chemistry, 14.05.2021 05:40 lescoto9035
Reaction 2:
10 Na, + 2 KNO
3 (s)
KO + 5 Nao +
2 (9)
Based on the mass of sodium produced from reaction 1, how many of grams of
nitrogen will also be produced in reaction 2?
+
Show your stoichiometry work in the tables below, including all appropriate units,
for full credit
gN
46 g Na
This slide is worth 2.5 pts total

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
Questions

French, 29.01.2020 17:46

French, 29.01.2020 17:46




Spanish, 29.01.2020 17:46


Mathematics, 29.01.2020 17:46

Mathematics, 29.01.2020 17:46

Biology, 29.01.2020 17:46

History, 29.01.2020 17:46

Geography, 29.01.2020 17:46

Mathematics, 29.01.2020 17:46

Mathematics, 29.01.2020 17:46

Mathematics, 29.01.2020 17:46

Spanish, 29.01.2020 17:46


History, 29.01.2020 17:46




