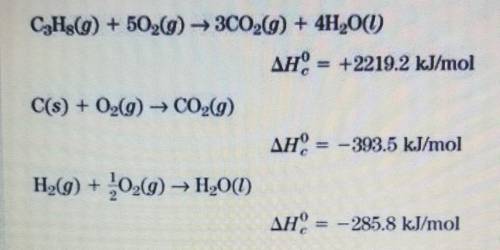Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
Use the equations below to calculate the enthalpy of formation for propane gas, C3H8, from its eleme...
Questions




Mathematics, 02.04.2020 18:20

Mathematics, 02.04.2020 18:20

English, 02.04.2020 18:20

English, 02.04.2020 18:20

Mathematics, 02.04.2020 18:20



Arts, 02.04.2020 18:21

Biology, 02.04.2020 18:21


Mathematics, 02.04.2020 18:21



Mathematics, 02.04.2020 18:22

Mathematics, 02.04.2020 18:22