
Chemistry, 13.05.2021 23:00 honeylebling
Consider the chemical equation. 2NBr3 + 3NaOH Right arrow. N2 + 3NaBr + 3HOBr If there are 40 mol of NBr3 and 48 mol of NaOH, what is the excess reactant?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

You know the right answer?
Consider the chemical equation. 2NBr3 + 3NaOH Right arrow. N2 + 3NaBr + 3HOBr If there are 40 mol of...
Questions

Mathematics, 01.03.2020 22:11

Mathematics, 01.03.2020 22:12

Mathematics, 01.03.2020 22:14


Mathematics, 01.03.2020 22:14

Social Studies, 01.03.2020 22:15

Social Studies, 01.03.2020 22:16

English, 01.03.2020 22:16

Computers and Technology, 01.03.2020 22:16

English, 01.03.2020 22:17


English, 01.03.2020 22:17

Mathematics, 01.03.2020 22:18


History, 01.03.2020 22:18


Mathematics, 01.03.2020 22:19


Computers and Technology, 01.03.2020 22:23

Mathematics, 01.03.2020 22:24

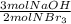 =60 moles NaOH
=60 moles NaOH

