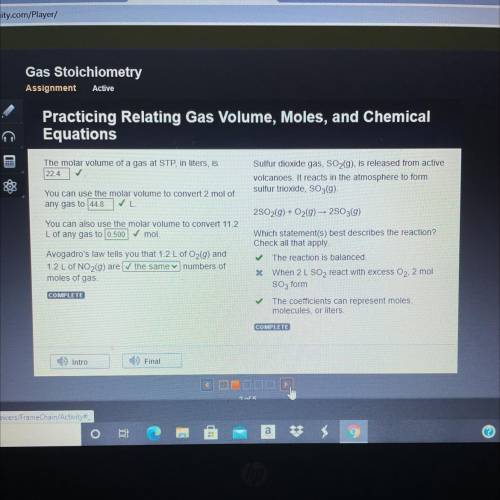
The molar volume of a gas at STP, in liters, is
22.4 ✓
You can use the molar volume to conver...

The molar volume of a gas at STP, in liters, is
22.4 ✓
You can use the molar volume to convert 2 mol of
any gas to 44.8
You can also use the molar volume to convert 11.2
L of any gas to 0.500
mol.
Avogadro's law tells you that 1.2 L of O2(9) and
1.2 L of NO2(9) are the same numbers of
moles of gas.
COMPLETE

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1


Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Questions

Biology, 17.12.2020 04:40


Mathematics, 17.12.2020 04:40

Mathematics, 17.12.2020 04:40





History, 17.12.2020 04:40


Mathematics, 17.12.2020 04:40

Biology, 17.12.2020 04:40

Chemistry, 17.12.2020 04:40

Mathematics, 17.12.2020 04:40

Computers and Technology, 17.12.2020 04:40

History, 17.12.2020 04:40

Physics, 17.12.2020 04:40

Computers and Technology, 17.12.2020 04:40

Mathematics, 17.12.2020 04:40



