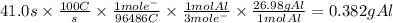Chemistry, 13.05.2021 20:50 naomicervero
In the Hall-Heroult process, a large electric current is passed through a solution of aluminum oxide Al2O3 dissolved in molten cryolite Na3AlF6, resulting in the reduction of the Al2O3 to pure aluminum. Suppose a current of 100.A is passed through a Hall-Heroult cell for 41.0 seconds. Calculate the mass of pure aluminum produced. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
In the Hall-Heroult process, a large electric current is passed through a solution of aluminum oxide...
Questions


















Computers and Technology, 06.12.2019 02:31