
Chemistry, 13.05.2021 18:10 Aracelys3946
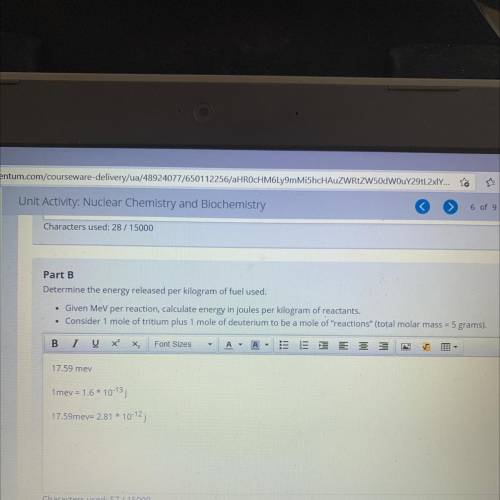
Part B
Determine the energy released per kilogram of fuel used.
• Given MeV per reaction, calculate energy in joules per kilogram of reactants.
• Consider 1 mole of tritium plus 1 mole of deuterium to be a mole of "reactions" (total molar mass = 5 grams).

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3
You know the right answer?
Part B
Determine the energy released per kilogram of fuel used.
• Given MeV per reaction, cal...
• Given MeV per reaction, cal...
Questions

Social Studies, 08.07.2019 07:20




Biology, 08.07.2019 07:20

Mathematics, 08.07.2019 07:20


Mathematics, 08.07.2019 07:20


Mathematics, 08.07.2019 07:20


Mathematics, 08.07.2019 07:20

Mathematics, 08.07.2019 07:20


Mathematics, 08.07.2019 07:20



Physics, 08.07.2019 07:20

Mathematics, 08.07.2019 07:20

Mathematics, 08.07.2019 07:20



