
Chemistry, 13.05.2021 03:30 hahalol123goaway
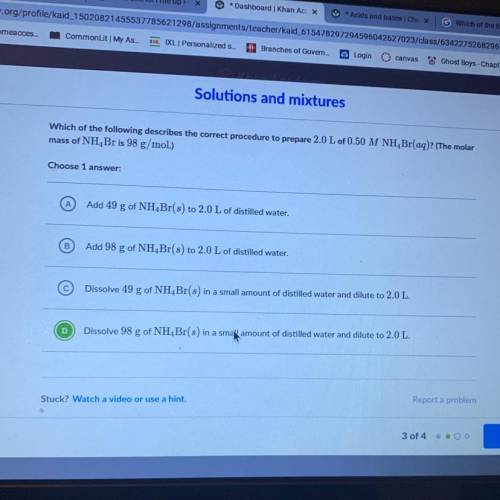
Which of the following describes the correct procedure to prepare 2.0 L of 0.50 M NH Br(aq)? (The molar
mass of NH, Br is 98 g/mol.)
Choose 1
BO
Add 49 g of NH Br(s) to 2.0 L of distilled water.
Add 98 g of NH Br(s) to 2.0 L of distilled water.
MY
Col
Dissolve 49 g of NH Br(s) in a small amount of distilled water and dilute to 2.0 L.
SA
Dissolve 98 g of NH Br(s) in a small amount of distilled water and dilute to 2.0 L.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Which of the following describes the correct procedure to prepare 2.0 L of 0.50 M NH Br(aq)? (The mo...
Questions



English, 13.10.2019 22:50

Mathematics, 13.10.2019 22:50

Physics, 13.10.2019 22:50



Mathematics, 13.10.2019 22:50


Mathematics, 13.10.2019 22:50


English, 13.10.2019 22:50

History, 13.10.2019 22:50

Mathematics, 13.10.2019 22:50

Business, 13.10.2019 22:50

Advanced Placement (AP), 13.10.2019 22:50


Mathematics, 13.10.2019 22:50

Social Studies, 13.10.2019 22:50



