
Chemistry, 13.05.2021 01:00 jasonr182017
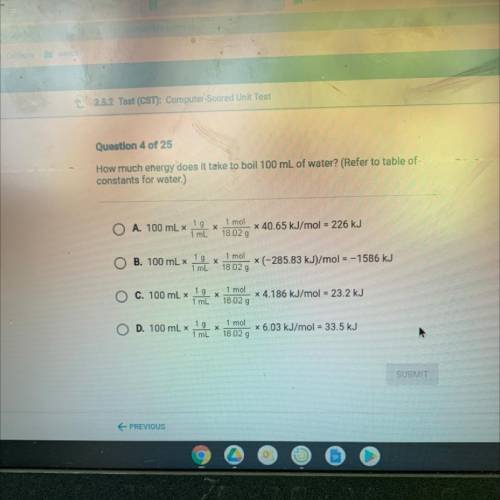
How much energy does it take to boil 100 mL of water? (Refer to table of
constants for water.)
A. 100 mL x
1 g
х
1 mL
1 mol
18.02 g
x 40.65 kJ/mol = 226 kJ
B. 100 mL x
1 g
1 mL
X
1 mol
18.02 g
(-285.83 kJ)/mol = –1586 kJ
C. 100 mL x
1 g
X
1 mol
18.02 g
x 4.186 kJ/mol = 23.2 kJ
1 mL
1 g
D. 100 mL x
1 mL
1 mol
x
18 02 g
x 6.03 kJ/mol = 33.5 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

You know the right answer?
How much energy does it take to boil 100 mL of water? (Refer to table of
constants for water.)
Questions

English, 20.12.2020 01:00

English, 20.12.2020 01:00

Mathematics, 20.12.2020 01:00


Physics, 20.12.2020 01:00





Mathematics, 20.12.2020 01:00

English, 20.12.2020 01:00


History, 20.12.2020 01:00



Business, 20.12.2020 01:00

Mathematics, 20.12.2020 01:00

Mathematics, 20.12.2020 01:00




