Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
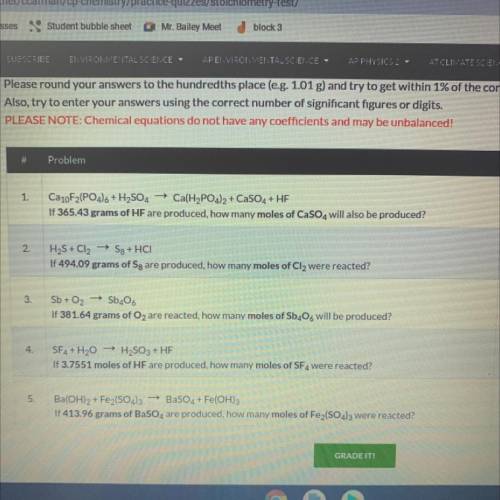
Ca10F2(PO4)6 + H2SO4 + Ca(H2PO4)2 + CaSO4 + HF
If 365.43 grams of HF are produced, how many moles o...
Questions

Physics, 13.01.2021 20:10




Mathematics, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10

History, 13.01.2021 20:10



History, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10

History, 13.01.2021 20:10




Mathematics, 13.01.2021 20:10


Mathematics, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10