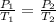Chemistry, 12.05.2021 18:20 asherkbohannan
A gas contained in a steel tank has a pressure of 1.5atm at a temperature of 320K. What will be the gas pressure when the temperature changes to 450K at a constant amount of gas and volume?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

You know the right answer?
A gas contained in a steel tank has a pressure of 1.5atm at a temperature of 320K. What will be the...
Questions


Mathematics, 01.12.2020 22:20






Mathematics, 01.12.2020 22:20


Geography, 01.12.2020 22:20

English, 01.12.2020 22:20



Mathematics, 01.12.2020 22:20

Mathematics, 01.12.2020 22:20


Mathematics, 01.12.2020 22:20