
Chemistry, 12.05.2021 17:00 tipbri6380
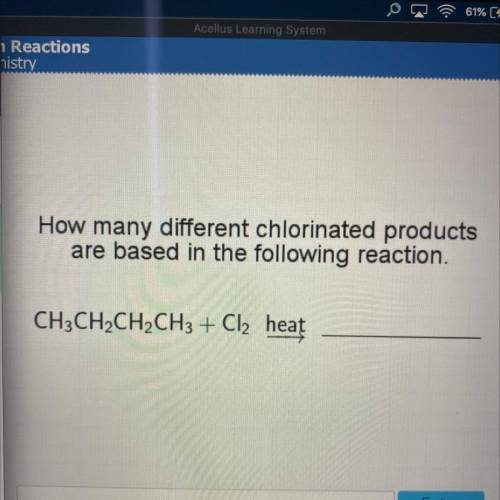
How many different chlorinated products
are based in the following reaction.
CH3CH2CH2CH3 + Cl2 heat

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 23.06.2019 10:00
Which element forms a compound with chlorine with the general formula mci?
Answers: 1
You know the right answer?
How many different chlorinated products
are based in the following reaction.
CH3CH2CH2CH3 + C...
CH3CH2CH2CH3 + C...
Questions


Mathematics, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31


English, 31.10.2019 23:31





History, 31.10.2019 23:31



Physics, 31.10.2019 23:31


English, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31




