
Chemistry, 12.05.2021 14:00 bg988763p7cl2d
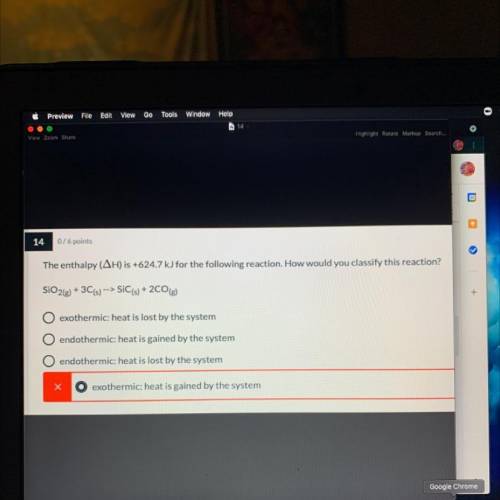
The enthalpy (AH) is +624.7 kJ for the following reaction. How would you classify this reaction? (Explain)
SiO2(g) + 3C(s) --> SIC(s) +2CO(g)
A. exothermic: heat is lost by the system
B. endothermic: heat is gained by the system
C. endothermic: heat is lost by the system
D. exothermic: heat is gained by the system

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

You know the right answer?
The enthalpy (AH) is +624.7 kJ for the following reaction. How would you classify this reaction? (Ex...
Questions

Mathematics, 22.04.2021 06:40


Mathematics, 22.04.2021 06:40

Social Studies, 22.04.2021 06:40


Mathematics, 22.04.2021 06:40


Mathematics, 22.04.2021 06:40



Mathematics, 22.04.2021 06:40


English, 22.04.2021 06:40

English, 22.04.2021 06:40


Mathematics, 22.04.2021 06:40


Mathematics, 22.04.2021 06:40


English, 22.04.2021 06:40

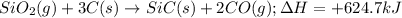 is classified as endothermic: heat is gained by the system.
is classified as endothermic: heat is gained by the system.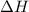 is positive which means heat is gained by the system.
is positive which means heat is gained by the system.

