
Chemistry, 12.05.2021 09:50 kaieshaweston
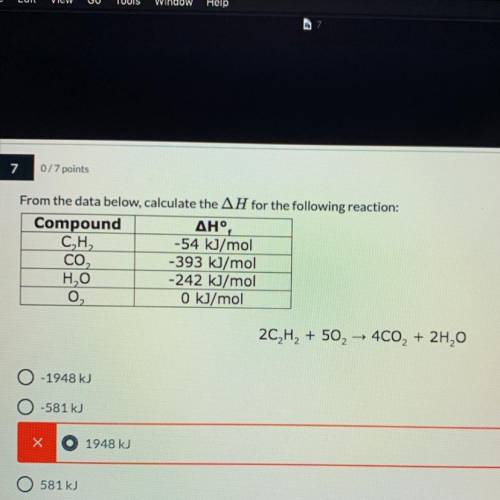
From the data below, calculate the AH for the following reaction:(need to know how you got the answer)
2C2H2 + 50, – 400, + 2H,0
A.- 1948 kJ
B.-581 kJ
C.1948 kJ
D.581 kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
From the data below, calculate the AH for the following reaction:(need to know how you got the answe...
Questions

Mathematics, 28.07.2019 16:20

Physics, 28.07.2019 16:20


English, 28.07.2019 16:20

Computers and Technology, 28.07.2019 16:20


English, 28.07.2019 16:20

Mathematics, 28.07.2019 16:20





English, 28.07.2019 16:30



English, 28.07.2019 16:30

Social Studies, 28.07.2019 16:30



English, 28.07.2019 16:30



