
Chemistry, 12.05.2021 09:40 weckesserj9492
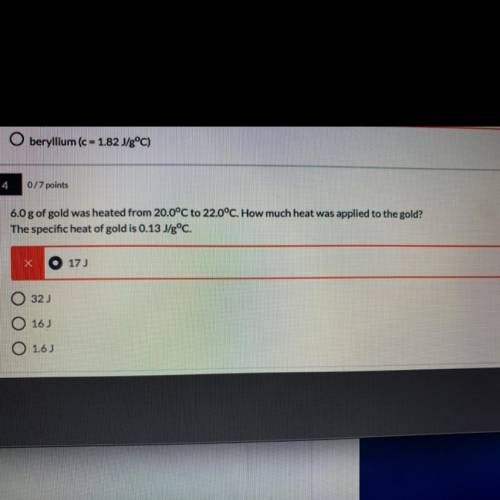
6.0 g of gold was heated from 20.0°C to 22.0°C. How much heat was applied to the gold?
The specific heat of gold is 0.13 J/gºC. (I need to explain why also )
A. 17J
B.32J
C.16J
D.1.6J

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
6.0 g of gold was heated from 20.0°C to 22.0°C. How much heat was applied to the gold?
The specific...
Questions


Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Biology, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

English, 17.03.2021 23:40

Chemistry, 17.03.2021 23:40

Arts, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40

Chemistry, 17.03.2021 23:40

Chemistry, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40


Social Studies, 17.03.2021 23:40




English, 17.03.2021 23:40




