X-29: 28.976 amu; 4.68%

Chemistry, 11.05.2021 23:10 andrewbigbrains8740
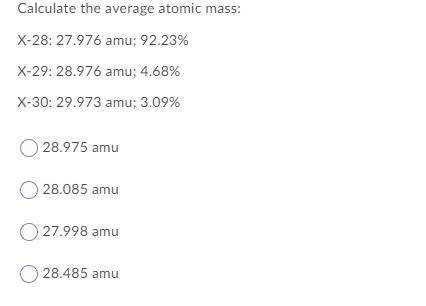
Calculate the average atomic mass:
X-28: 27.976 amu; 92.23%
X-29: 28.976 amu; 4.68%
X-30: 29.973 amu; 3.09%

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Calculate the average atomic mass:
X-28: 27.976 amu; 92.23%
X-29: 28.976 amu; 4.68%
X-29: 28.976 amu; 4.68%
Questions

Biology, 20.09.2019 20:30



Mathematics, 20.09.2019 20:30

Mathematics, 20.09.2019 20:30


Social Studies, 20.09.2019 20:30




Biology, 20.09.2019 20:30


Mathematics, 20.09.2019 20:30


Biology, 20.09.2019 20:30

Mathematics, 20.09.2019 20:30



Social Studies, 20.09.2019 20:30



