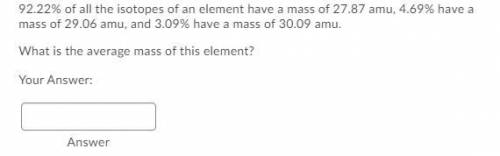Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

You know the right answer?
92.22% of all the isotopes of an element have a mass of 27.87 amu, 4.69% have a mass of 29.06 amu, a...
Questions



Computers and Technology, 08.12.2020 01:10


Health, 08.12.2020 01:10

Mathematics, 08.12.2020 01:10

French, 08.12.2020 01:10

Arts, 08.12.2020 01:10



Mathematics, 08.12.2020 01:10




Mathematics, 08.12.2020 01:10



Physics, 08.12.2020 01:10


Mathematics, 08.12.2020 01:10