
Chemistry, 11.05.2021 20:20 alexiagreen1212
Al2S3->Al+S If 1000g of aluminum sulfide decomposes, how many moles of aluminum should be produced?
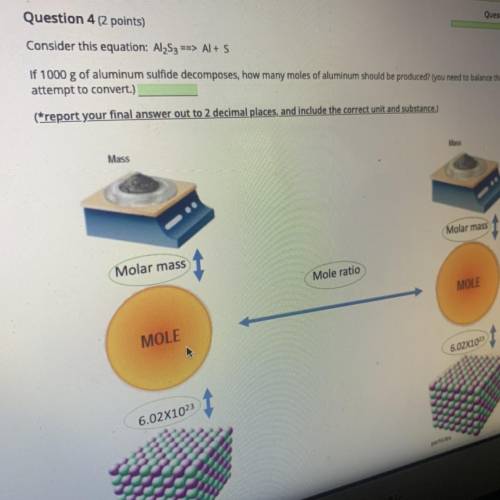

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2


Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Al2S3->Al+S
If 1000g of aluminum sulfide decomposes, how many moles of aluminum should be produc...
Questions


Mathematics, 01.03.2021 20:40

French, 01.03.2021 20:40

Biology, 01.03.2021 20:40



Arts, 01.03.2021 20:40

Mathematics, 01.03.2021 20:40

Mathematics, 01.03.2021 20:40

History, 01.03.2021 20:40

Mathematics, 01.03.2021 20:40

Health, 01.03.2021 20:40




Mathematics, 01.03.2021 20:40



English, 01.03.2021 20:40

Mathematics, 01.03.2021 20:40



