
Chemistry, 11.05.2021 18:50 1r32tgy5hk7
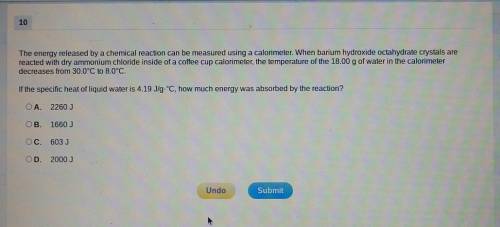
The energy released by a chemical reaction can be measured using a calorimeter. When barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00g of water in the calorimeter decreases from 30.0⁰C to 8.0⁰C. If the specific heat of liquid water is 4.19J/g.⁰C, how much energy was absorbed by the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

You know the right answer?
The energy released by a chemical reaction can be measured using a calorimeter. When barium hydroxid...
Questions

English, 10.11.2020 05:10

Mathematics, 10.11.2020 05:10

Mathematics, 10.11.2020 05:10



Mathematics, 10.11.2020 05:10

Mathematics, 10.11.2020 05:10

Mathematics, 10.11.2020 05:10


Mathematics, 10.11.2020 05:10

Mathematics, 10.11.2020 05:10


Mathematics, 10.11.2020 05:10

English, 10.11.2020 05:10


Mathematics, 10.11.2020 05:10

Mathematics, 10.11.2020 05:10


Mathematics, 10.11.2020 05:10

Mathematics, 10.11.2020 05:10



