Consider the energy diagram below.
AY
B
Free Energy
Reaction Progression
Wh...

Chemistry, 11.05.2021 17:10 ruchierosanp1n3qw
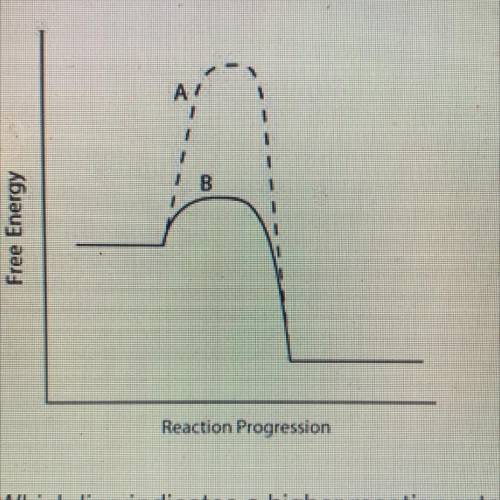
Consider the energy diagram below.
AY
B
Free Energy
Reaction Progression
Which line indicates a higher reaction rate?
A because it has a lower activation energy.
B because it has a lower activation energy.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Questions




Mathematics, 18.02.2020 22:05

Mathematics, 18.02.2020 22:05

English, 18.02.2020 22:05



Mathematics, 18.02.2020 22:05

English, 18.02.2020 22:05

Health, 18.02.2020 22:05


Mathematics, 18.02.2020 22:05






Biology, 18.02.2020 22:05

Mathematics, 18.02.2020 22:05



