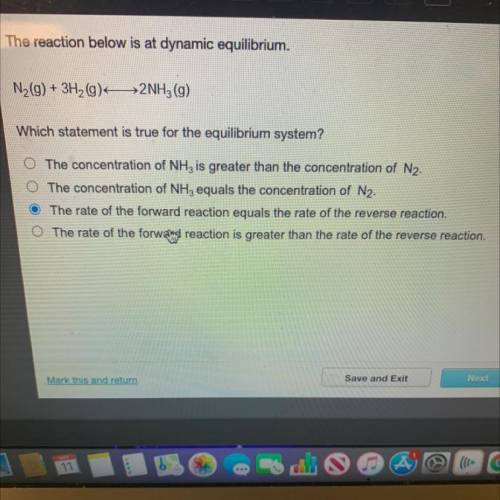
N(9) + 3H2(9) 2NH3 (9)
Which statement is true for the equilibrium system?
The concentration...

N(9) + 3H2(9) 2NH3 (9)
Which statement is true for the equilibrium system?
The concentration of NH, is greater than the concentration of N2.
O The concentration of NH3 equals the concentration of N2.
The rate of the forward reaction equals the rate of the reverse reaction.
O The rate of the forwald reaction is greater than the rate of the reverse reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3
You know the right answer?
Questions




Business, 13.12.2019 09:31

Mathematics, 13.12.2019 09:31






Social Studies, 13.12.2019 09:31

Mathematics, 13.12.2019 09:31

Mathematics, 13.12.2019 09:31

English, 13.12.2019 09:31


Mathematics, 13.12.2019 09:31


Mathematics, 13.12.2019 09:31


English, 13.12.2019 09:31



