
Chemistry, 10.05.2021 21:30 adreyan3479
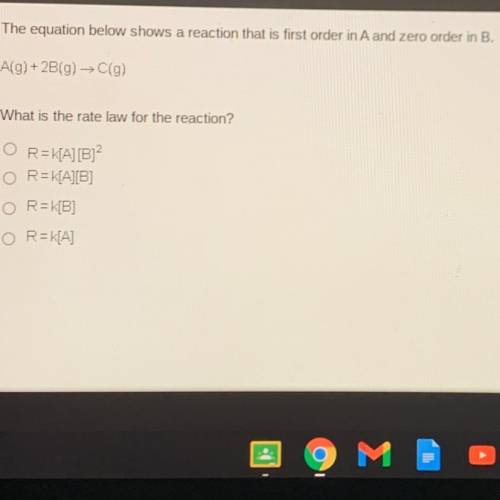
The equation below shows a reaction that is first order in A and zero order in B.
A(g) + 2B(g) →C(g)
What is the rate law for the reaction?
R=K[A] [B]2
R=K[A][B]
R=K[B]
R=K[A]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
The equation below shows a reaction that is first order in A and zero order in B.
A(g) + 2B(g) →C(...
Questions

Physics, 02.10.2019 17:10

Mathematics, 02.10.2019 17:10


History, 02.10.2019 17:10

Mathematics, 02.10.2019 17:10

Social Studies, 02.10.2019 17:10


Mathematics, 02.10.2019 17:10

English, 02.10.2019 17:10

History, 02.10.2019 17:10

Mathematics, 02.10.2019 17:10


History, 02.10.2019 17:10









