
Chemistry, 10.05.2021 14:00 amiechap12
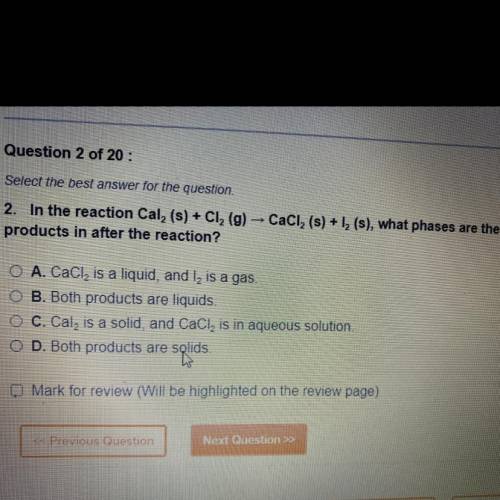
In the reaction Cal 2 (s)+Cl 2 (g) CaCl 2 (s)+l 2 (s) , what phases are the products in after the reaction ?
A. CaCl 2 is a liquid, and l2 is a gas
B. Both products are a liquid
C. Cal2 is a solid, CaCl2 is in aqueous solution
D. Both products are solids

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
In the reaction Cal 2 (s)+Cl 2 (g) CaCl 2 (s)+l 2 (s) , what phases are the products in after the re...
Questions

Biology, 01.10.2019 19:30

Computers and Technology, 01.10.2019 19:30

History, 01.10.2019 19:30

World Languages, 01.10.2019 19:30






English, 01.10.2019 19:30


Mathematics, 01.10.2019 19:30


English, 01.10.2019 19:30


History, 01.10.2019 19:30




Advanced Placement (AP), 01.10.2019 19:30



