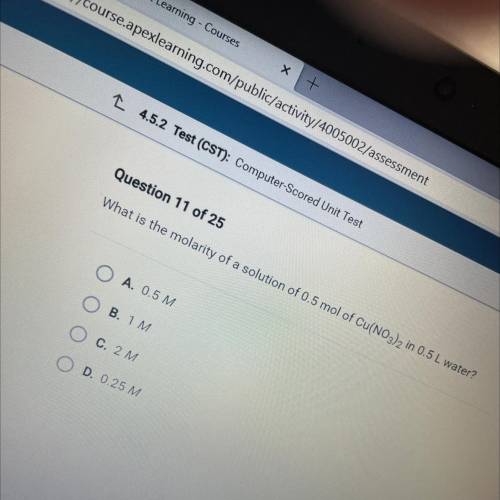
What is the molarity of a solution of 0.5 mol of Cu(NO3)2 in 0.5 L water?
...

Chemistry, 10.05.2021 01:40 lucystudies
What is the molarity of a solution of 0.5 mol of Cu(NO3)2 in 0.5 L water?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Questions

Social Studies, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20

History, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20







Mathematics, 09.12.2020 23:20


English, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20


Mathematics, 09.12.2020 23:20




