PLEASE HELP!!
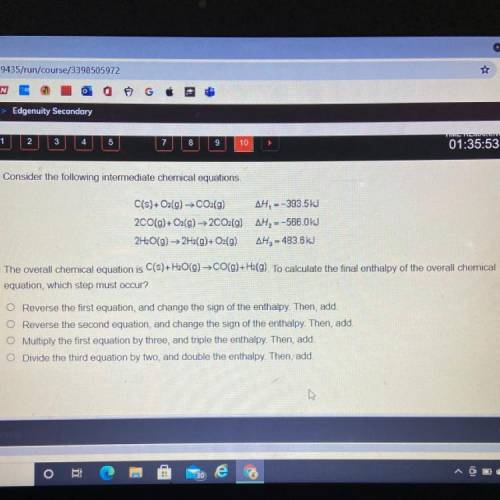
Consider the following intermediate chemical equations.
C(s) + O2(g) → CO2(g)...

Chemistry, 09.05.2021 21:40 jaydahh4059
PLEASE HELP!!
Consider the following intermediate chemical equations.
C(s) + O2(g) → CO2(g) AH, --393.5kJ
2CO(g) + O2(g) → 2CO2(g) AH, --566.0kJ
2H2O(g) → 2H2(g)+O2(g) AH2 - 483.6 kJ
The overall chemical equation is C(s) + H2O(g) → CO(g) +H2(g). To calculate the final enthalpy of the overall chemica
equation, which step must occur?
Reverse the first equation, and change the sign of the enthalpy. Then, add.
O Reverse the second equation, and change the sign of the enthalpy. Then, add.
O Multiply the first equation by three, and triple the enthalpy. Then, add.
O Divide the third equation by two, and double the enthalpy. Then, add.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
Questions









Computers and Technology, 29.08.2019 21:20


Social Studies, 29.08.2019 21:20



Biology, 29.08.2019 21:30


Mathematics, 29.08.2019 21:30

Mathematics, 29.08.2019 21:30



Mathematics, 29.08.2019 21:30



