
Chemistry, 08.05.2021 22:50 lerasteidl
How many moles of carbon dioxide are produced in the reaction between hydrochloric acid and calcium carbonate when 2 moles of HCl are used to start with?
2HCl + CaCO3 +CaCl2 + CO2 + H2O
A. 2
B. 4
C. 1
D. 3
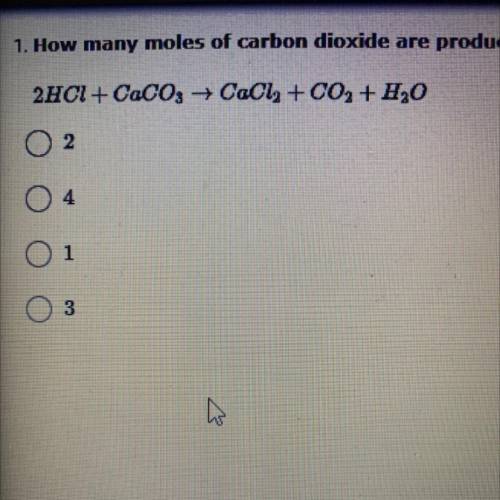

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
How many moles of carbon dioxide are produced in the reaction between hydrochloric acid and calcium...
Questions



Mathematics, 24.11.2020 14:00

Health, 24.11.2020 14:00

Mathematics, 24.11.2020 14:00

Advanced Placement (AP), 24.11.2020 14:00

Social Studies, 24.11.2020 14:00

Computers and Technology, 24.11.2020 14:00

Social Studies, 24.11.2020 14:00


Business, 24.11.2020 14:00



Biology, 24.11.2020 14:00

Computers and Technology, 24.11.2020 14:00

Social Studies, 24.11.2020 14:00

History, 24.11.2020 14:00


English, 24.11.2020 14:00

English, 24.11.2020 14:00




