
Chemistry, 07.05.2021 22:30 aallyssabrown0120
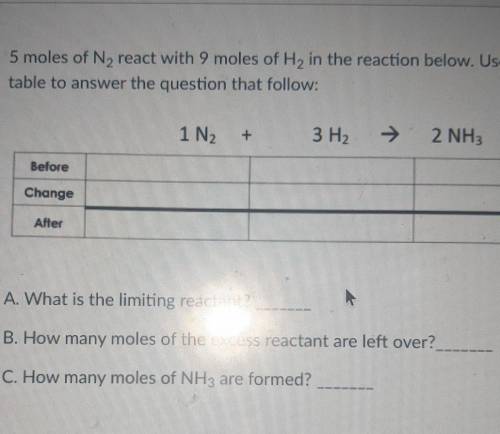
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the question that follow: 1N2+3H2=2NH3
A. What is the limiting reactant?
B. How many moles of the excess reactant are left over?
C. How many moles of NH3 are formed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the questi...
Questions

Mathematics, 14.05.2021 19:20

Social Studies, 14.05.2021 19:20

Mathematics, 14.05.2021 19:20

Health, 14.05.2021 19:20



Mathematics, 14.05.2021 19:20


Computers and Technology, 14.05.2021 19:20

Mathematics, 14.05.2021 19:20

Biology, 14.05.2021 19:20

Mathematics, 14.05.2021 19:20


Mathematics, 14.05.2021 19:20

Physics, 14.05.2021 19:20



Chemistry, 14.05.2021 19:20

History, 14.05.2021 19:20




