
Chemistry, 07.05.2021 22:20 keananashville
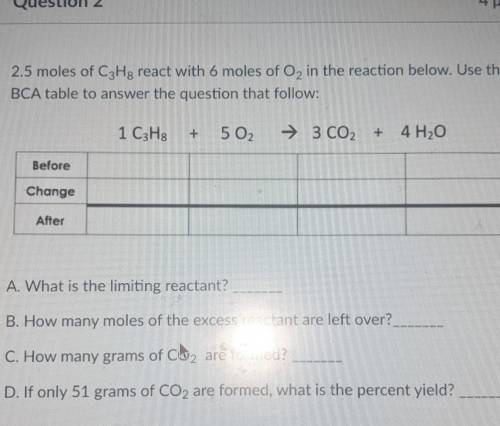
2.5 moles of C3H8 react with 6 moles of O2 in the reaction below. Use the BCA table to answer the question that follow: 1 C3H8 + 5 02 → 3 CO2 + 4H2O
A. What is the limiting reactant?
B. How many moles of the excess reactant are left over?.
C. How many grams of CO2 are formed?
D. If only 51 grams of CO2 are formed, what is the percent yield?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1

Chemistry, 23.06.2019 08:00
Match the vocabulary terms to their definitions. 1 . a long, chain-like set of molecules made up of repeating units joined end to end polymer 2 . a hard, brittle, heat- and corrosion-resistant material made by subjecting a nonmetallic mineral mixture to intense heat ceramic 3 . a plastic with low elongations that cannot be recycled thermoset 4 . a carbon fiber embedded in a polymer resin matrix thermoplastic 5 . a plastic with high elongations that can be recycled crystal 6 . a solid form resulting from the arrangement of atoms, ions, or molecules in definite geometric patterns composite
Answers: 1

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
2.5 moles of C3H8 react with 6 moles of O2 in the reaction below. Use the BCA table to answer the qu...
Questions



Mathematics, 21.01.2021 02:40



Biology, 21.01.2021 02:40

History, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40

English, 21.01.2021 02:40




Mathematics, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40

English, 21.01.2021 02:40

History, 21.01.2021 02:40


Mathematics, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40



