
Chemistry, 07.05.2021 14:00 ThetPerson
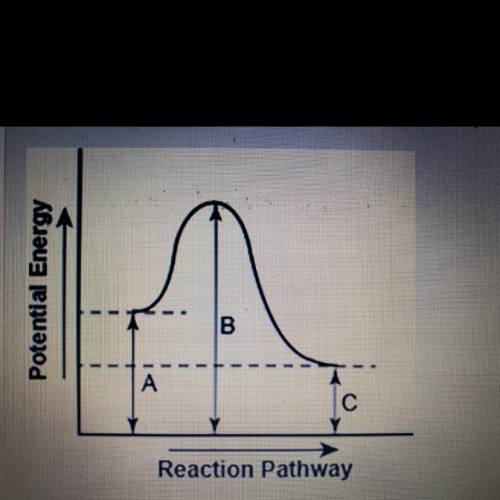
The diagram shows the potential energy changes for a reaction pathway.
Part 1: does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer.
Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1


Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway.
Part 1: does the diagram il...
Questions

Mathematics, 13.02.2021 23:40

Advanced Placement (AP), 13.02.2021 23:40

Mathematics, 13.02.2021 23:40




English, 13.02.2021 23:40

Mathematics, 13.02.2021 23:40

Mathematics, 13.02.2021 23:40

Chemistry, 13.02.2021 23:40

Chemistry, 13.02.2021 23:40


Mathematics, 13.02.2021 23:40





Spanish, 13.02.2021 23:40

Mathematics, 13.02.2021 23:40



