
Chemistry, 07.05.2021 14:00 tmcdowell69
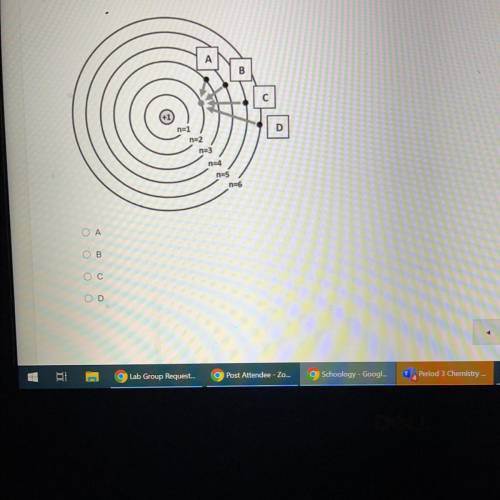
Which of the electron movements shown below (A, B, C, or D) is most likely to produce a red-colored light?
B
D
na3
n24
n25
n=6
Ο Α
OB
Ос
OD

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2
You know the right answer?
Which of the electron movements shown below (A, B, C, or D) is most likely to produce a red-colored...
Questions








English, 18.10.2019 22:00











History, 18.10.2019 22:00

Biology, 18.10.2019 22:00



