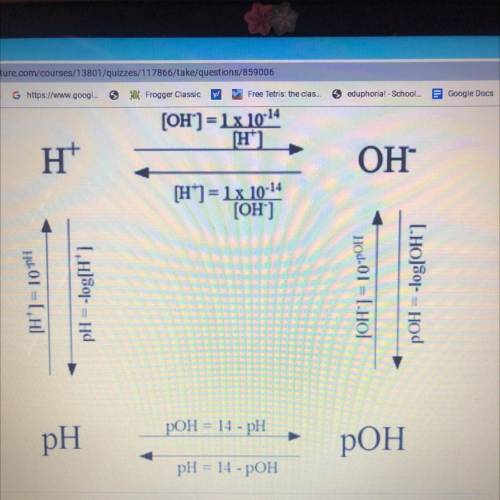
What is the pH of an aqueous solution with a hydrogen ion concentration of 1.8x10^-4M?
...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
Questions

English, 05.05.2020 06:53







Mathematics, 05.05.2020 06:53




Social Studies, 05.05.2020 06:53

Mathematics, 05.05.2020 06:54

History, 05.05.2020 06:54

Mathematics, 05.05.2020 06:54


Mathematics, 05.05.2020 06:54

Mathematics, 05.05.2020 06:54