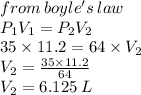Chemistry, 06.05.2021 21:00 angel213326
A gas occupies 11.2 L at a pressure of 35.0 mm . What is the volume of the gas if it is Increased to a pressure of 64.0 mm Hg?
A) 6.13 L
B) 110.2 L
C) 200.0 K
D) 20.5 L

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
A gas occupies 11.2 L at a pressure of 35.0 mm . What is the volume of the gas if it is Increased to...
Questions


English, 03.02.2020 11:49

Mathematics, 03.02.2020 11:49


Mathematics, 03.02.2020 11:49



Arts, 03.02.2020 11:49

Health, 03.02.2020 11:49

English, 03.02.2020 11:49


English, 03.02.2020 11:49

History, 03.02.2020 11:49

Mathematics, 03.02.2020 11:49

Mathematics, 03.02.2020 11:49

Arts, 03.02.2020 11:49



History, 03.02.2020 11:49

Chemistry, 03.02.2020 11:49