
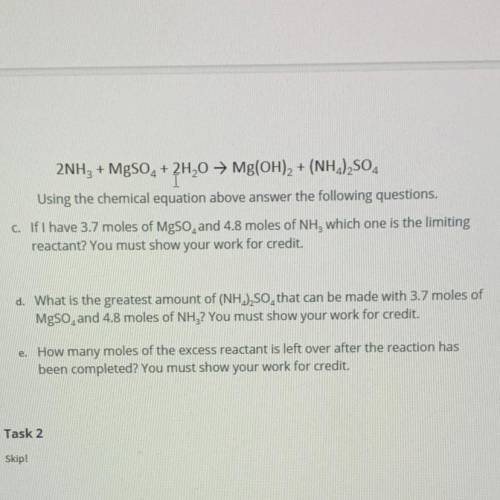
2NH3 + MgSO2 + 2H20 → Mg(OH)2 + (NH2)2SO4
Using the chemical equation above answer the following questions.
c. If I have 3.7 moles of MgSO, and 4.8 moles of NH, which one is the limiting
reactant? You must show your work for credit.
d. What is the greatest amount of (NH.),SO, that can be made with 3.7 moles of
MgSO, and 4.8 moles of NH3? You must show your work for credit.
e. How many moles of the excess reactant is left over after the reaction has
been completed? You must show your work for credit.
Please show work it would be greatly appreciated

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
2NH3 + MgSO2 + 2H20 → Mg(OH)2 + (NH2)2SO4
Using the chemical equation above answer the following q...
Questions












Business, 29.08.2019 04:10








Computers and Technology, 29.08.2019 04:10



