
Chemistry, 05.05.2021 23:20 bryanmcmillianjr
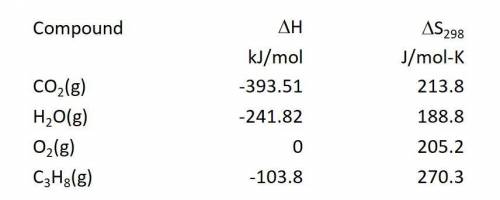
Calculate the Gibbs free energy change in kJ/mol at 25.0 C for the reaction: C3H8(g) + 5O2(g) ---> 3CO2(g) + 4H2O(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1
You know the right answer?
Calculate the Gibbs free energy change in kJ/mol at 25.0 C for the reaction: C3H8(g) + 5O2(g) --->...
Questions





Mathematics, 08.07.2021 20:40

Geography, 08.07.2021 20:40



Social Studies, 08.07.2021 20:40


Biology, 08.07.2021 20:40


Mathematics, 08.07.2021 20:40

Mathematics, 08.07.2021 20:40

Mathematics, 08.07.2021 20:40




English, 08.07.2021 20:40



