
Chemistry, 05.05.2021 21:50 Aliyahh5673
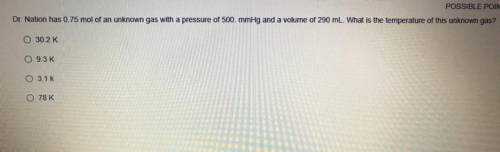
Dr. Nation has 0.75 mol of an unknown gas with a pressure of 500 mmHg and a volume of 290 mL. What is the temperature of this unknown gas?
0 30.2 K
93K
3.1K
O 78 K

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1



Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Dr. Nation has 0.75 mol of an unknown gas with a pressure of 500 mmHg and a volume of 290 mL. What i...
Questions



Mathematics, 27.10.2020 21:10

Health, 27.10.2020 21:10

Computers and Technology, 27.10.2020 21:10

Mathematics, 27.10.2020 21:10

History, 27.10.2020 21:10




English, 27.10.2020 21:10



Mathematics, 27.10.2020 21:10

Business, 27.10.2020 21:10

Computers and Technology, 27.10.2020 21:10

English, 27.10.2020 21:10


Geography, 27.10.2020 21:10



