
Chemistry, 05.05.2021 21:40 jasperzhouzihe3018
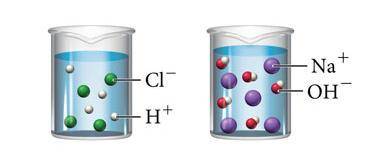
The image above shows what happens when hydrochloric acid (HCl) and sodium hydroxide (NaOH) are mixed with water. Which statement below is true about both solutions?
A. Both solutions contain solutes that do not dissolve completely in water.
B. Both solutions are saturated because they contain the maximum concentration of a solute dissolved in the solvent.
C. Both solutions will conduct an electric current because they both contain ions that can carry a charge.
D. Both solutions are acidic because they both contain hydrogen.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3


Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
The image above shows what happens when hydrochloric acid (HCl) and sodium hydroxide (NaOH) are mixe...
Questions

Mathematics, 14.04.2021 03:40

Mathematics, 14.04.2021 03:40


Mathematics, 14.04.2021 03:40



Physics, 14.04.2021 03:40

Mathematics, 14.04.2021 03:40


Mathematics, 14.04.2021 03:40


Mathematics, 14.04.2021 03:40

Mathematics, 14.04.2021 03:40


Mathematics, 14.04.2021 03:40

History, 14.04.2021 03:40



English, 14.04.2021 03:40

Mathematics, 14.04.2021 03:40



