

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3
You know the right answer?
4. Balance the following equation. Then determine the number of mols of Nitrogen formed given the va...
Questions


English, 23.03.2021 03:10


Mathematics, 23.03.2021 03:10

Social Studies, 23.03.2021 03:10




Mathematics, 23.03.2021 03:10

Mathematics, 23.03.2021 03:10


Mathematics, 23.03.2021 03:10

Mathematics, 23.03.2021 03:10



English, 23.03.2021 03:10

English, 23.03.2021 03:10

Biology, 23.03.2021 03:10


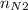 = 4 × 2 ÷ 4 = 2
= 4 × 2 ÷ 4 = 2 

